- Authors
-
- Author
- Super Admin
- Published on
Demam Berdarah Dengue (DBD)
Diseases Name
Demam Berdarah Dengue (DBD)
Diseases Cure
1. Obat penurun demam, 2. Istirahat yang banyak di tempat tidur, 3. Minum banyak cairan
lab check
Enzym Linked Immunosorbent Assay (ELISA)
diseases prevention
Kenakan pakaian tertutup saat bepergian, terutama di sore hari Kenakan lotion anti nyamuk Lakukan langkah 3M (menguras penampungan air, mengubur, dan mendaur ulang barang bekas) untuk membasmi sarang nyamuk Semprot lingkungan Anda dengan gas fogging
Symptoms
Sakit kepala parah, nyeri pada otot, tulang dan sendi, mual, muntah, kelenjar bengkak, ruam pada kulit
medicine
Paracetamol dan Transfusi trombosit
diagnosis
Untuk diagnosis pasti, tes darah demam berdarah juga akan diperlukan.
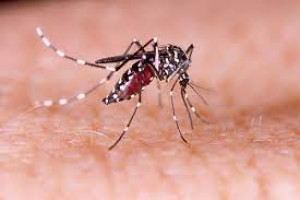
INFECTIOUS AGENT
Dengue, an acute febrile illness, is caused by infection with any of 4 related positive-sense, single-stranded RNA viruses of the genus Flavivirus, dengue viruses 1, 2, 3, or 4.
TRANSMISSION
Almost all transmission occurs through the bite of infected Aedes mosquitoes, primarily Aedes aegypti and Ae. albopictus. Because of the approximately 7-day viremia in humans, bloodborne transmission is possible through exposure to infected blood, organs, or other tissues (such as bone marrow). In addition, perinatal dengue transmission occurs when the mother is infected near the time of birth, in which infection occurs via microtransfusions when the placenta is detached or through mucosal contact with mother’s blood during birth. Congenital transmission has not been documented. Dengue viruses may also be transmitted through breast milk. There is no evidence of sexual transmission.
EPIDEMIOLOGY
Dengue is endemic throughout the tropics and subtropics and is a leading cause of febrile illness among travelers returning from Latin America, the Caribbean, and Southeast Asia. Dengue occurs in >100 countries worldwide (Maps 4-01 through 4-03), including Puerto Rico, the US Virgin Islands, and US-affiliated Pacific Islands. Sporadic outbreaks with local transmission have occurred in Florida, Hawaii, and Texas along the border with Mexico. Although the geographic distribution of dengue is similar to that of malaria, dengue is more of a risk in urban and residential areas than is malaria. DengueMap (www.healthmap.org/dengue/index.php) shows up-to-date information on areas of ongoing transmission.
Map 4-01. Dengue risk in the Americas and the Caribbean
Risk areas are shown on a national level except for where evidence exists of different risk levels at subnational regions. Areas that are too small to be seen on the regional maps are labeled in dark blue or light blue depending on their risk categorization.
Map 4-02. Dengue risk in Africa, Europe, and the Middle East
Risk areas are shown on a national level except for where evidence exists of different risk levels at subnational regions. Areas that are too small to be seen on the regional maps are labeled in dark blue or light blue depending on their risk categorization.
Map 4-03. Dengue risk in Asia and Oceania
CLINICAL PRESENTATION
An estimated 40%–80% of infections are asymptomatic. Symptomatic infection (dengue) most commonly presents as a mild to moderate, nonspecific, acute febrile illness. However, as many as 5% of all dengue patients develop severe, life-threatening disease. Early clinical findings are nonspecific but require a high index of suspicion, because recognizing early signs of shock and promptly initiating intensive supportive therapy can reduce risk of death among patients with severe dengue by at least 20-fold to <0.5%. See Box 4-02 for information regarding the World Health Organization guidelines for classifying dengue.
Dengue begins abruptly after an incubation period of 5–7 days (range, 3–10 days), and the course follows 3 phases: febrile, critical, and convalescent. Fever typically lasts 2–7 days and can be biphasic. Other signs and symptoms may include severe headache; retroorbital pain; muscle, joint, and bone pain; macular or maculopapular rash; and minor hemorrhagic manifestations, including petechiae, ecchymosis, purpura, epistaxis, bleeding gums, hematuria, or a positive tourniquet test result. Some patients have an injected oropharynx and facial erythema in the first 24–48 hours after onset. Warning signs of progression to severe dengue occur in the late febrile phase around the time of defervescence and include persistent vomiting, severe abdominal pain, fluid accumulation, mucosal bleeding, difficulty breathing, lethargy/restlessness, postural hypotension, liver enlargement, and progressive increase in hematocrit (hemoconcentration).
The critical phase of dengue begins at defervescence and typically lasts 24–48 hours. Most patients clinically improve during this phase, but those with substantial plasma leakage develop severe dengue as a result of a marked increase in vascular permeability. Initially, physiologic compensatory mechanisms maintain adequate circulation, which narrows pulse pressure as diastolic blood pressure increases. Patients with severe plasma leakage have pleural effusions or ascites, hypoproteinemia, and hemoconcentration. Patients may appear well despite early signs of shock. However, once hypotension develops, systolic blood pressure rapidly declines, and irreversible shock and death may ensue despite resuscitation efforts. Patients can also develop severe hemorrhagic manifestations, including hematemesis, bloody stool, melena, or menorrhagia, especially if they have prolonged shock. Uncommon manifestations include hepatitis, myocarditis, pancreatitis, and encephalitis.
As plasma leakage subsides, the patient enters the convalescent phase and begins to reabsorb extravasated intravenous fluids and pleural and abdominal effusions. As a patient’s well-being improves, hemodynamic status stabilizes (although he or she may manifest bradycardia), and diuresis ensues. The patient’s hematocrit stabilizes or may fall because of the dilutional effect of the reabsorbed fluid, and the white cell count usually starts to rise, followed by a recovery of the platelet count. The convalescent-phase rash may desquamate and be pruritic.
Laboratory findings commonly include thrombocytopenia, hyponatremia, elevated aspartate aminotransferase and alanine aminotransferase, and a normal erythrocyte sedimentation rate.
Data are limited on health outcomes of dengue in pregnancy and effects of maternal infection on the developing fetus. Perinatal transmission can occur, and peripartum maternal infection may increase the likelihood of symptomatic infection in the newborn. Of 41 perinatal transmission cases described in the literature, all developed thrombocytopenia, most had evidence of plasma leakage evidenced by ascites or pleural effusions, and fever was absent in only 2. Nearly 40% had a hemorrhagic manifestation, and one-fourth had hypotension. Symptoms in perinatally infected neonates typically present during the first week of life. Placental transfer of maternal IgG against dengue virus (from a previous maternal infection) may increase risk for severe dengue among infants infected at 6–12 months of age when the protective effect of these antibodies wanes.
Box 4-02. Guidelines for classifying dengue
In November 2009, the World Health Organization (WHO) issued a new guideline that classifies symptomatic cases as dengue or severe dengue.
Dengue is defined by a combination of ≥2 clinical findings in a febrile person who traveled to or lives in a dengue-endemic area. Clinical findings include nausea, vomiting, rash, aches and pains, a positive tourniquet test, leukopenia, and the following warning signs: abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy, restlessness, liver enlargement, and postural hypotension. The presence of a warning sign may predict severe dengue in a patient.
Severe dengue is defined by dengue with any of the following symptoms: severe plasma leakage leading to shock or fluid accumulation with respiratory distress; severe bleeding; or severe organ impairment such as elevated transaminases ≥1,000 IU/L, impaired consciousness, or heart impairment.
From 1975 through 2009, symptomatic dengue virus infections were classified according to the WHO guidelines as dengue fever, dengue hemorrhagic fever (DHF), and dengue shock syndrome (the most severe form of DHF). The case definition was changed to the 2009 clinical classification after reports that the case definition of DHF was both too difficult to apply in resource-limited settings and too specific, as it failed to identify a substantial proportion of severe dengue cases, including cases of hepatic failure and encephalitis. The 2009 clinical classification has been criticized for being overly inclusive, as it allows several different ways to qualify for severe dengue, and nonspecific warning signs are used as diagnostic criteria for dengue.
DIAGNOSIS
Clinicians should consider dengue in a patient who was in an endemic area within 2 weeks of symptom onset. Because dengue is a nationally notifiable disease, all suspected cases should be reported to the state or local health department. Laboratory confirmation can be made from a single acute-phase serum specimen obtained early (≤7 days after fever onset) in the illness by detecting viral genomic sequences with RT-PCR or dengue nonstructural protein 1 (NS1) antigen by immunoassay. Later in the illness (≥4 days after fever onset), IgM against dengue virus can be detected with ELISA. For patients presenting during the first week after fever onset, diagnostic testing should include a test for dengue virus (RT-PCR or NS1) and IgM (Figure 4-01). For patients presenting >1 week after fever onset, an IgM test is most useful. In the United States, both IgM ELISA and real-time RT-PCR are approved as in vitro diagnostic tests.
Presence of virus by RT-PCR or NS1 antigen in a single diagnostic specimen is considered laboratory confirmation of dengue in patients with a compatible clinical and travel history. IgM in a single serum sample suggests a probable recent dengue infection and should be considered diagnostic for dengue if the infection most likely occurred in a place where other potentially cross-reactive flaviviruses (such as Zika, West Nile, yellow fever, and Japanese encephalitis viruses) are not a risk. If infection is likely to have occurred in a place where other potentially cross-reactive flaviviruses circulate, both molecular and serologic diagnostic testing should be performed to detect evidence of infection with dengue and the other flaviviruses.
IgG by ELISA in a single serum sample is not useful for routine diagnostic testing because it remains detectable for life after infection. In addition, people infected with or vaccinated against other flaviviruses (such as yellow fever or Japanese encephalitis) may produce cross-reactive flavivirus antibodies, yielding false-positive serologic dengue diagnostic test results.
Dengue diagnostic testing (molecular and serologic) is available from several commercial reference diagnostic laboratories, state public health laboratories, and CDC (www.cdc.gov/Dengue/clinicalLab/index.html). Consultation on dengue diagnostic testing can be obtained from CDC at 787-706-2399.
Figure 4-01. Relative sensitivity of detection of dengue virus nucleic acid, antigen, and IgM1
Abbreviations: DENV, dengue virus; NS1, nonstructural protein 1.
1 DENV RNA and NS1 are detectable during the first week of illness. Anti-DENV IgM is detectable starting approximately 5 days after illness onset. Although most cases only have detectable IgM anti-DENV for 14–20 days after illness onset, in some cases it may be detectable for up to 90 days. Detection of anti-DENV IgG is neither sensitive nor specific in identifying patients with dengue.
TREATMENT
No specific antiviral agents exist for dengue. Patients should be advised to stay well hydrated and to avoid aspirin (acetylsalicylic acid), aspirin-containing drugs, and other nonsteroidal anti- inflammatory drugs (such as ibuprofen) because of their anticoagulant properties. Fever should be controlled with acetaminophen and tepid sponge baths. Febrile patients should avoid mosquito bites to reduce risk of further transmission. For those who develop severe dengue, close observation and frequent monitoring in an intensive care unit setting may be required. Prophylactic platelet transfusions in dengue patients are not beneficial and may contribute to fluid overload. Similarly, administration of corticosteroids has no demonstrated benefit and is potentially harmful to patients; corticosteroids should not be used except in the case of autoimmune-related complications.
PREVENTION
A vaccine to prevent dengue (Dengvaxia) has been licensed in almost 20 countries and approved for commercial use in 11 countries. However, in late 2017, the vaccine manufacturer, Sanofi Pasteur, announced that people who receive the vaccine and have not been previously infected with a dengue virus may be at risk of developing more severe manifestations of dengue. The Philippines suspended its dengue vaccine program, and other countries have specified that only people who have been infected with a dengue virus should receive the vaccine. Two other dengue vaccines are currently in phase 3 clinical trials, for which completion is expected in 2018 and 2019.
No prophylaxis is available to prevent dengue. Risk increases with duration of travel and disease incidence in the travel destination (such as during the rainy season and during epidemics). Travelers going to the tropics for any length of time should avoid mosquito bites by taking the following preventive measures:
- Select accommodations with well-screened windows and doors or air conditioning when possible. Aedes mosquitoes typically live indoors and are often found in dark, cool places, such as in closets, under beds, behind curtains, in bathrooms, and on porches.
- Wear clothing that covers the arms and legs, especially during the early morning and late afternoon, when risk of being bitten is the highest.
- Use insect repellent (see Chapter 3, Mosquitoes, Ticks & Other Arthropods).
- For longer-term travelers, empty and clean or cover any standing water that can be mosquito-breeding sites in the local residence (such as water storage tanks or flowerpots).
CDC website: www.cdc.gov/dengue
BIBLIOGRAPHY
- Arragain L, Dupont-Rouzeyrol M, O’Connor O, Sigur N, Grangeon JP, Huguon E, et al. Vertical transmission of dengue virus in the peripartum period and viral kinetics in newborns and breast milk: new data. J Pediatric Infect Dis. 2017 Nov 24;6(4):324–31.
- Clapham HE, Cummings DAT, Johansson MA. Immune status alters the probability of apparent illness due to dengue virus infection: evidence from a pooled analysis across multiple cohort and cluster studies. PLoS Negl Trop Dis. 2017 Sep 27;11(9):e0005926.
- Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010 Dec;8(12 Suppl):S7–16.
- Lam PK, Tam DT, Diet TV, Tam CT, Tien NT, Kieu NT, et al. Clinical characteristics of dengue shock syndrome in Vietnamese children: a 10-year prospective study in a single hospital. Clin Infect Dis. 2013 Dec;57(11):1577–86.
- Leder K, Torresi J, Libman MD, Cramer JP, Castelli F, Schlagenhauf P, et al. GeoSentinel surveillance of illness in returned travelers, 2007–2011. Ann Intern Med. 2013 Mar 19;158(6):456–68.
- Schwartz E, Weld LH, Wilder-Smith A, von Sonnenburg F, Keystone JS, Kain KC, et al. Seasonality, annual trends, and characteristics of dengue among ill returned travelers, 1997–2006. Emerg Infect Dis. 2008 Jul;14(7):1081–8.
- Simmons CP, Farrar JJ, van Vinh Chau N, Wills B. Dengue. N Engl J Med. 2012 Apr 12;366(15):1423–32.
- Srikiatkhachorn A, Rothman AL, Gibbons RV, Sittisombut N, Malasit P, Ennis FA, et al. Dengue—how best to classify it. Clin Infect Dis. 2011 Sep;53(6):563–7.
- Stanaway JD, Shepard DS, Undurraqa EA, Halasa YA, Coffeng LE, Brady OJ. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016 Jun;16(6):712–23.
- Tomashek KM, Margolis HS. Dengue: a potential transfusion-transmitted disease. Transfusion. 2011 Aug;51(8):1654–60.
- World Health Organization. Dengue guidelines for diagnosis, treatment, prevention and control: new edition. Geneva: World Health Organization; 2009. [cited 2019 Apr 1]. Available from: www.who.int/rpc/guidelines/9789241547871/en/.
Dilihat 100 kali
diperbarui pada 01 November 2022
Anda bisa ikut berdiskusi dengan membuat pertanyaan dibawah ini
Daftar Pertanyaan
Pertanyaan dari: zxc
asd
asd
Pertanyaan dari: sweety
contoh pertanyaan
contoh pertanyaan
Dijawab oleh:
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.